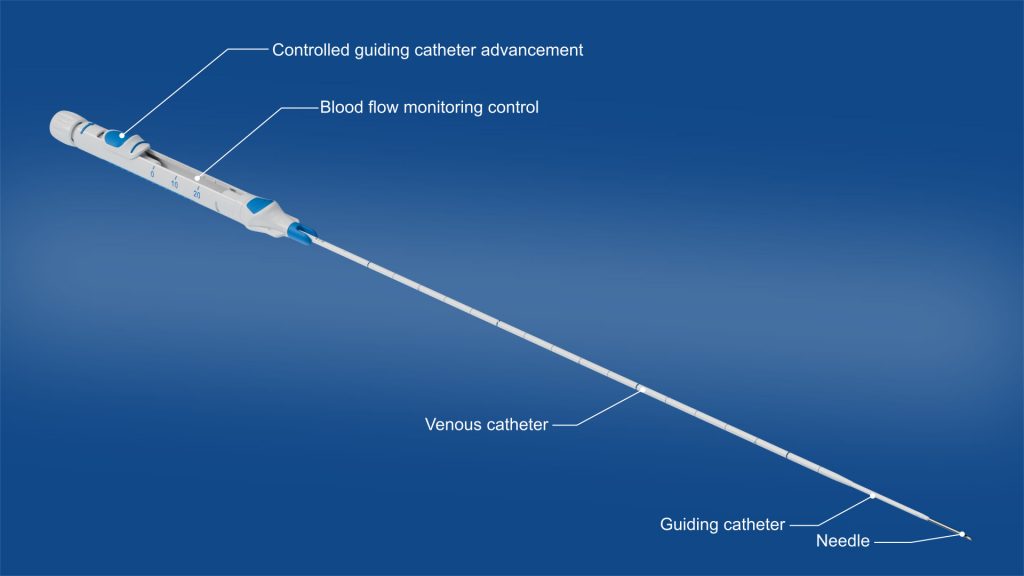
Midline Catheter (not approved for commercial distribution)
A midline catheter is a small, flexible venous access device placed in the upper arm to deliver intravenous medications over several days up to multiple weeks.
Due to its longer dwell time, a midline catheter is more patient-friendly than repeated peripheral venous punctures. At the same time, it is less invasive than a central venous catheter, and is associated with a lower complication rate (1), particularly due to:
- Placement outside the central venous system (no risk of pneumothorax) (2)
- Significantly reduced risk of severe catheter-related bloodstream infections (3)
- Lower training and resource requirements for insertion procedure (4)
- No x-ray exposure, as no radiographic tip confirmation is required
Expected Improvements
- For intuitive application
- Technology to reduce infection risks
- Patient- and user-centered design
The Ebnet Medical Midline Catheter is being designed as a platform technology for various medical applications and is currently under development. The product is not approved for commercial distribution.
The objective is to demonstrate a positive correlation between the technical features of the system and measurably improved patient outcomes.
Method
Schematic representation of the method; not approved for use in patients.
Product not approved for commercial distribution.
Why Midlines Are Becoming Increasingly Popular
- The use of midline catheters reduces the need for central venous catheters or repeated placement of peripheral intravenous cannulas (3).
- Reduction of resources and costs, as well as prevention of catheter failure (1,3,4,5,6,7)
- High market share in the U.S. market (8)
Extensive Patent Portfolio
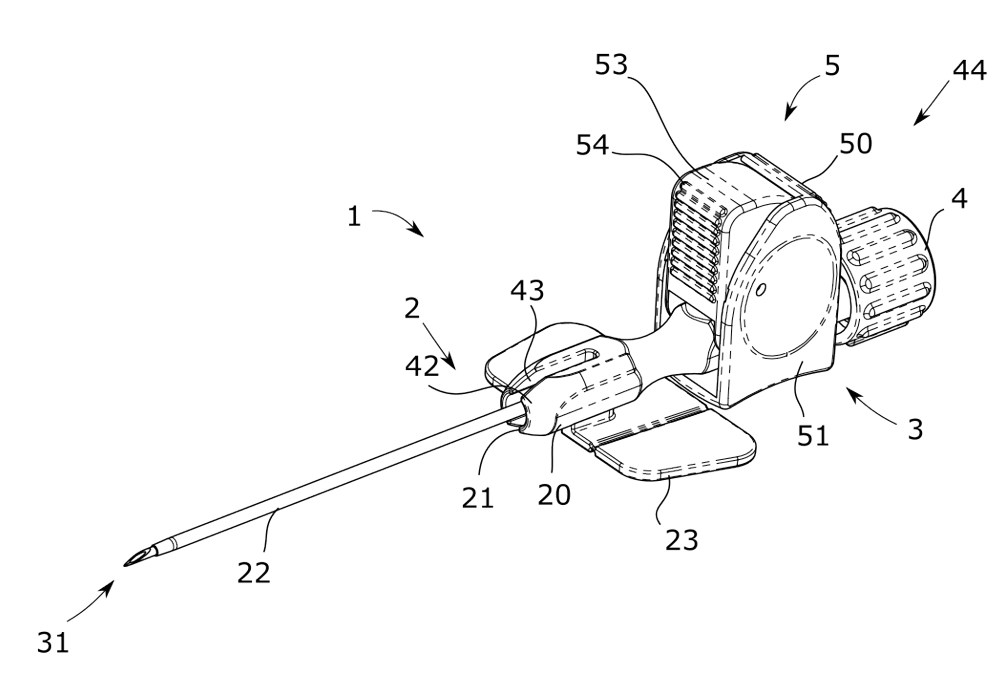
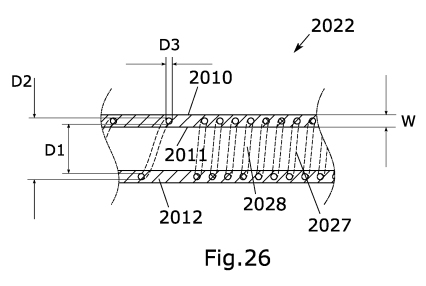
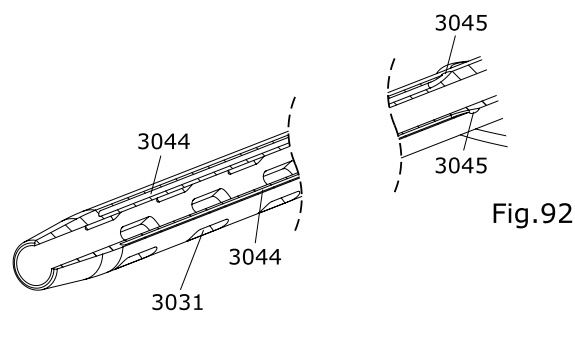
20 2018 101 646.6 (Deutschland, Gebrauchsmusterschutz)
DE 10 2021 115 847 A1 (Deutschland, angemeldet)
PCT/EP2019/057097 (internationale Patentanmeldung)
PCT/EP2020/087404 (internationale Patentanmeldung)
PCT/EP2022/066380 (internationale Patentanmeldung)
PCT/EP2024/081180 (internationale Patentanmeldung)
19713743.3 (EU, angemeldet)
20842219.6 (EU, angemeldet)
17/040,342 (USA, angemeldet)
17/786,835 (USA, angemeldet)
201980028955.9 (China, angemeldet)
202080095919.7 (China, angemeldet)
202037041537 (Indien, erteilt)
2020-550830 (Japan, angemeldet)
Zusätzliche Schutzrechte angemeldet (noch nicht veröffentlicht, Optionen für zahlreiche nationale/regionale Anmeldungen)
References
- Adams DZ, Little A, Vinsant C, Khandelwal S. The midline catheter: a clinical review. Journal of Emergency Medicine. 2016;51(3):252-258, 05Jan2026, 18:05
- Tsotsolis N, Tsirgogianni K, Kioumis I, Pitsiou G, Baka S, Papaiwannou A, Karavergou A, Rapti A, Trakada G, Katsikogiannis N, Tsakiridis K, Karapantzos I, Karapantzou C, Barbetakis N, Zissimopoulos A, Kuhajda I, Andjelkovic D, Zarogoulidis K, Zarogoulidis P. Pneumothorax as a complication of central venous catheter insertion. Ann Transl Med. 2015 Mar;3(3):40. doi: 10.3978/j.issn.2305-5839.2015.02.11. PMID: 25815301; PMCID: PMC4356862. 05Jan2026, 18:04
- Pathak R, Gangina S, Jairam F, Hinton K. A vascular access and midlines program can decrease hospital-acquired central line-associated bloodstream infections and cost to a community-based hospital. Ther Clin Risk Manag. 2018 Aug 21;14:1453-1456. doi: 10.2147/TCRM.S171748. PMID: 30174427; PMCID: PMC6110272. 05Jan2025, 18:19
- Nielsen EB, et al. The efficacy of midline catheters—a prospective, randomized, active-controlled study. International Journal of Infectious Diseases. 2021;102:220–225; 05Jan2026, 14:02
- Kleidon TM, et al. Midline Compared With Peripheral Intravenous Catheters for Therapy of 4 Days or Longer in Pediatric Patients: A Randomized Clinical Trial. JAMA Pediatrics. 2023. 05Jan2026, 14:20
- Meto E, et al. Cost comparison of four venous catheters: Short peripheral catheter, Long peripheral line, Midline, and PICC for peripheral infusion. J Vasc Access. 2025 May;26(3):966–974. 05Jan2026, 14:36
- Gala S, et al. Evaluation of Cost Analysis of the Midline Catheter Versus Peripherally Inserted Central Catheter… 2020. 05Jan2026, 14:25
- Grand View Research. Midline Catheters Market Size, Share & Trends Report. 2023–2024. 05Jan2026, 14:12
Midline Catheter (not approved for commercial distribution)
Product not approved for commercial distribution.
A midline catheter is a small, flexible venous access device placed in the upper arm to deliver intravenous medications over several days up to multiple weeks.
Due to its longer dwell time, a midline catheter is more patient-friendly than repeated peripheral venous punctures. At the same time, it is less invasive than a central venous catheter, and is associated with a lower complication rate (1), particularly due to:
- Placement outside the central venous system (no risk of pneumothorax) (2)
- Significantly reduced risk of severe catheter-related bloodstream infections (3)
- Lower training and resource requirements for insertion procedure (4)
- No x-ray exposure, as no radiographic tip confirmation is required
Expected Improvements
- For intuitive application
- Technology to reduce infection risks
- Patient- and user-centered design
The Ebnet Medical Midline Catheter is being designed as a platform technology for various medical applications and is currently under development. The product is not approved for commercial distribution.
The objective is to demonstrate a positive correlation between the technical features of the system and measurably improved patient outcomes.
Method
Schematic representation of the method; not approved for use in patients.
Product not approved for commercial distribution.
Why Midlines Are Becoming Increasingly Popular
- The use of midline catheters reduces the need for central venous catheters or repeated placement of peripheral intravenous cannulas (3).
- Reduction of resources and costs, as well as prevention of catheter failure (1,3,4,5,6,7)
- High market share in the U.S. market (8)
Extensive Patent Portfolio



20 2018 101 646.6 (Deutschland, Gebrauchsmusterschutz)
DE 10 2021 115 847 A1 (Deutschland, angemeldet)
PCT/EP2019/057097 (internationale Patentanmeldung)
PCT/EP2020/087404 (internationale Patentanmeldung)
PCT/EP2022/066380 (internationale Patentanmeldung)
PCT/EP2024/081180 (internationale Patentanmeldung)
19713743.3 (EU, angemeldet)
20842219.6 (EU, angemeldet)
17/040,342 (USA, angemeldet)
17/786,835 (USA, angemeldet)
201980028955.9 (China, angemeldet)
202080095919.7 (China, angemeldet)
202037041537 (Indien, erteilt)
2020-550830 (Japan, angemeldet)
Zusätzliche Schutzrechte angemeldet (noch nicht veröffentlicht, Optionen für zahlreiche nationale/regionale Anmeldungen)
References
- Adams DZ, Little A, Vinsant C, Khandelwal S. The midline catheter: a clinical review. Journal of Emergency Medicine. 2016;51(3):252-258, 05Jan2026, 18:05
- Tsotsolis N, Tsirgogianni K, Kioumis I, Pitsiou G, Baka S, Papaiwannou A, Karavergou A, Rapti A, Trakada G, Katsikogiannis N, Tsakiridis K, Karapantzos I, Karapantzou C, Barbetakis N, Zissimopoulos A, Kuhajda I, Andjelkovic D, Zarogoulidis K, Zarogoulidis P. Pneumothorax as a complication of central venous catheter insertion. Ann Transl Med. 2015 Mar;3(3):40. doi: 10.3978/j.issn.2305-5839.2015.02.11. PMID: 25815301; PMCID: PMC4356862. 05Jan2026, 18:04
- Pathak R, Gangina S, Jairam F, Hinton K. A vascular access and midlines program can decrease hospital-acquired central line-associated bloodstream infections and cost to a community-based hospital. Ther Clin Risk Manag. 2018 Aug 21;14:1453-1456. doi: 10.2147/TCRM.S171748. PMID: 30174427; PMCID: PMC6110272. 05Jan2025, 18:19
- Nielsen EB, et al. The efficacy of midline catheters—a prospective, randomized, active-controlled study. International Journal of Infectious Diseases. 2021;102:220–225; 05Jan2026, 14:02
- Kleidon TM, et al. Midline Compared With Peripheral Intravenous Catheters for Therapy of 4 Days or Longer in Pediatric Patients: A Randomized Clinical Trial. JAMA Pediatrics. 2023. 05Jan2026, 14:20
- Meto E, et al. Cost comparison of four venous catheters: Short peripheral catheter, Long peripheral line, Midline, and PICC for peripheral infusion. J Vasc Access. 2025 May;26(3):966–974. 05Jan2026, 14:36
- Gala S, et al. Evaluation of Cost Analysis of the Midline Catheter Versus Peripherally Inserted Central Catheter… 2020. 05Jan2026, 14:25
- Grand View Research. Midline Catheters Market Size, Share & Trends Report. 2023–2024. 05Jan2026, 14:12