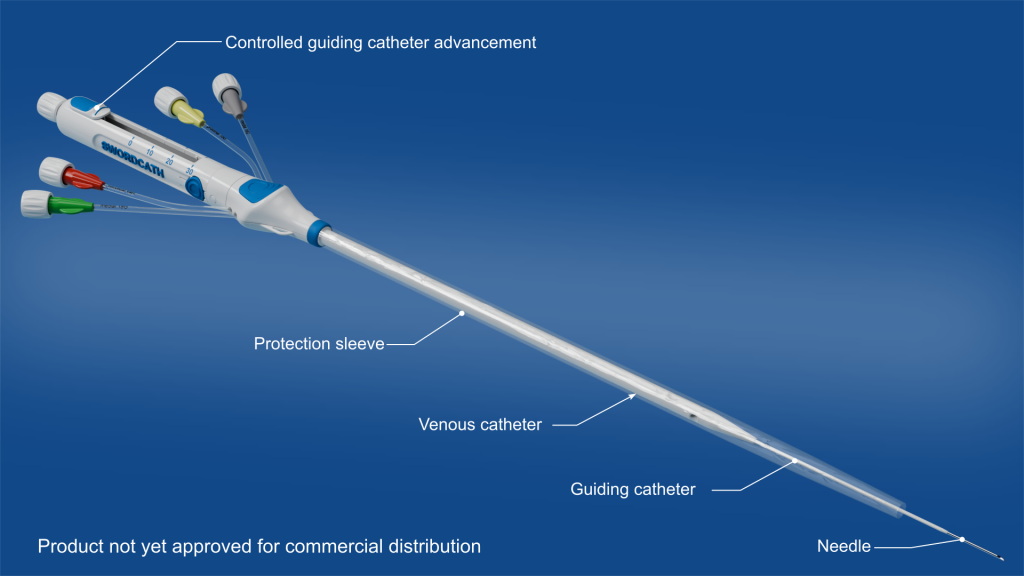
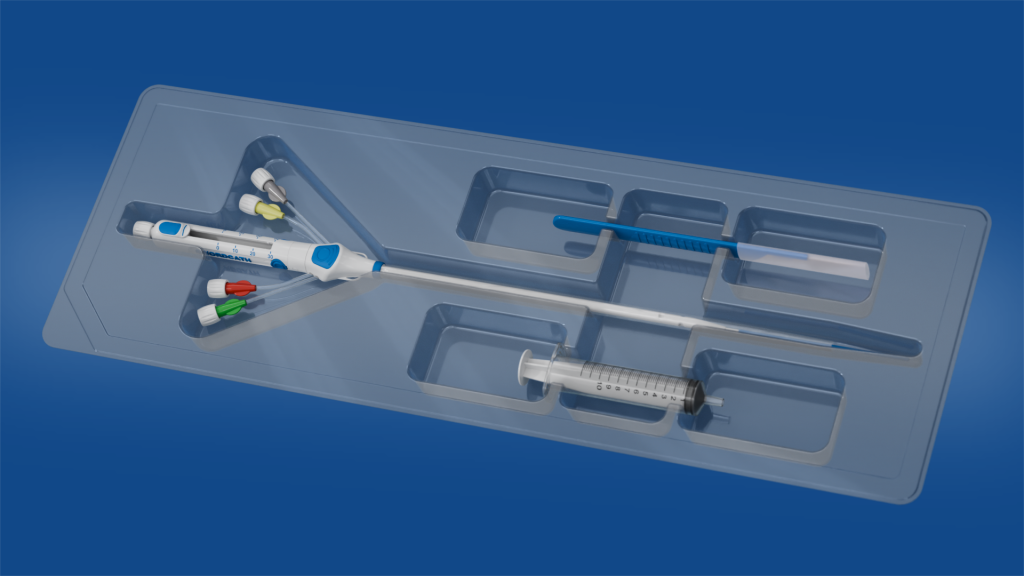
SwordCath® without Guidewire (not approved for commercial distribution)

- rapid administration of fluids and medications, particularly when administration via a peripheral access is not possible (1, 2)
- administration of highly concentrated or vessel-irritating substances (2)
- diagnostic purposes
- reliable efficacy of administered substances, independent of peripheral vasoconstriction or shock (1, 2)
- lower risk of tissue damage compared to peripheral access devices
- longer dwell time compared to peripheral access devices (3)
Expected Improvements
- For intuitive and safe access to larger veins
- Replacement of the Seldinger guidewire technique: thanks to its guiding catheter, the SwordCath® will not require Seldinger technique and therefore no guidewire
- The SwordCath® will be a ready-to-use product (not approved for commercial distribution) with all components integrated
- Aspirate-and-push concept for continuous monitoring of blood return and correct catheter positioning
- Placement possible without ultrasound in emergency medicine, as well as for the medical care of injured persons in high-risk situations
- Can be combined with ultrasound in appropriate situations, e.g., acute care or dialysis
The multi-patented SwordCath® is currently under development. It represents a revolutionary platform technology, designed to simplify and improve insertion of larger catheters into the body, particularly into blood vessels.
Unlike the traditional Seldinger technique, which has been used for decades and still carries its inherent complexities, SwordCath® (not approved for commercial distribution) will offer an alternative, eliminating the need for extensive training. Resource consumption and costly waste will be reduced.
With millions of procedures performed annually, it is time for an innovation in emergency medicine that can reduce complications, accelerate interventions, and at the same time enhance patient safety.
The system is designed as a platform technology for various medical applications and is currently under development. The product is not approved for commercial distribution.
The goal is to demonstrate a positive correlation between the system’s technical features and measurably improved patient outcomes.
Method
Schematic representation of the method; not approved for use in patients.
The product is not approved for commercial distribution.
Extensive Intellectual Property Portfolio
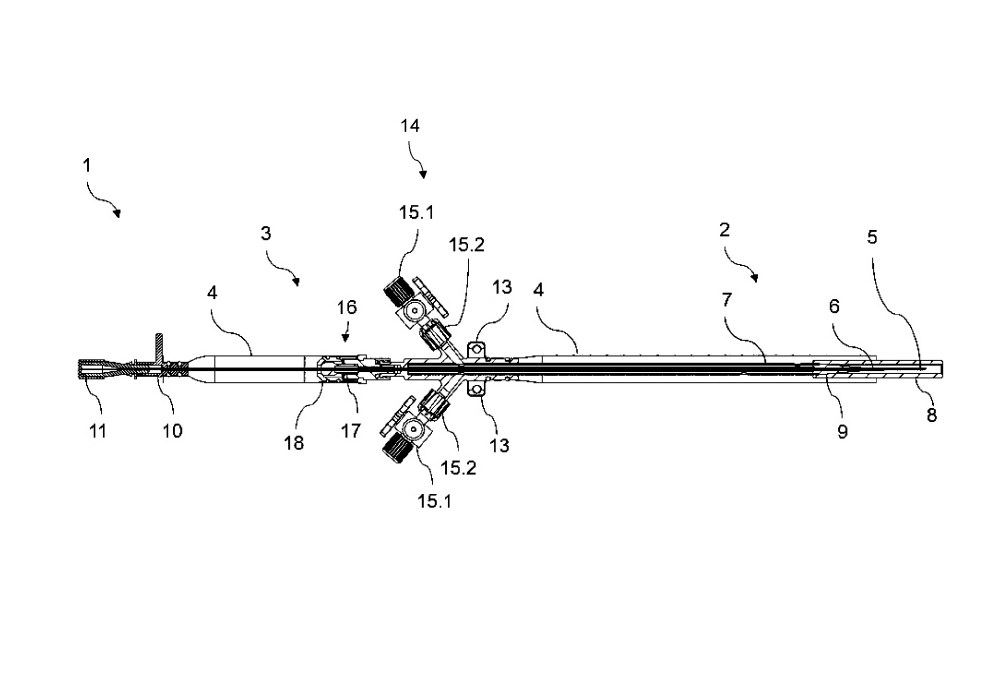
US 10,806,902 B2 (US, granted)
US 11,744,611 B2 (US, granted)
US 11,957,849 B2 (US, granted)
EP 3 328 478 B1 (EU, granted)
EP 3 746 167 B1 (EU, granted)
CN 111655321 B (China, granted)
AU 2016299343 B2 (Australia, granted)
AU 2019215680 B2 (Australia, granted)
BR 112018001895 B8 (Brazil, granted)
BR 112020015371 A8 (Brazil, granted)
422876 (India, granted)
547215 (India, granted)
JP 7315915 B2 (Japan, granted)
JP 7313698 B2 (Japan, granted)
PCT/EP2016/067785 (international patent application)
PCT/EP2019/051751 (international patent application)
201680044512.5 (China, pending)
References
- Barsky D, Radomislensky I, Talmy T, Gendler S, Almog O, Avital G. Association Between Profound Shock Signs and Peripheral Intravenous Access Success Rates in Trauma Patients in the Prehospital Scenario: A Retrospective Study. Anesth Analg. 2023 May 1;136(5):934-940. doi: 10.1213/ANE.0000000000006342. Epub 2023 Apr 14. PMID: 37058730. (18 Jan 2026; 14:45)
- Aldujeli A, Haq A, Tecson KM, Kurnickaite Z, Lickunas K, Bailey S, Tatarunas V, Braukyliene R, Baksyte G, Aldujeili M, Khalifeh H, Briedis K, Ordiene R, Unikas R, Hamadeh A, Brilakis ES. A prospective observational study on impact of epinephrine administration route on acute myocardial infarction patients with cardiac arrest in the catheterization laboratory (iCPR study). Crit Care. 2022 Dec 20;26(1):393. doi: 10.1186/s13054-022-04275-8. PMID: 36539907; PMCID: PMC9764590. (18. Jan. 2026, 15:00)
- https://www.cdc.gov/infection-control/hcp/intravascular-catheter-related-infections/summary-recommendations.html (18 Jan 2026, 18:19)Summary of Recommendations | Infection Control | CDC
SwordCath® without Guidewire (not approved for commercial distribution)

- rapid administration of fluids and medications, particularly when administration via a peripheral access is not possible (1, 2)
- administration of highly concentrated or vessel-irritating substances (2)
- diagnostic purposes
- reliable efficacy of administered substances, independent of peripheral vasoconstriction or shock (1, 2)
- lower risk of tissue damage compared to peripheral access devices
- longer dwell time compared to peripheral access devices (3)
Expected Improvements
- For intuitive and safe access to larger veins
- Replacement of the Seldinger guidewire technique: thanks to its guiding catheter, the SwordCath® will not require Seldinger technique and therefore no guidewire
- The SwordCath® will be a ready-to-use product (not approved for commercial distribution) with all components integrated
- Aspirate-and-push concept for continuous monitoring of blood return and correct catheter positioning
- Placement possible without ultrasound in emergency medicine, as well as for the medical care of injured persons in high-risk situations
- Can be combined with ultrasound in appropriate situations, e.g., acute care or dialysis
The multi-patented SwordCath® is currently under development. It represents a revolutionary platform technology, designed to simplify and improve insertion of larger catheters into the body, particularly into blood vessels.
Unlike the traditional Seldinger technique, which has been used for decades and still carries its inherent complexities, SwordCath® (not approved for commercial distribution) will offer an alternative, eliminating the need for extensive training. Resource consumption and costly waste will be reduced.
With millions of procedures performed annually, it is time for an innovation in emergency medicine that can reduce complications, accelerate interventions, and at the same time enhance patient safety.
The system is designed as a platform technology for various medical applications and is currently under development. The product is not approved for commercial distribution.
The goal is to demonstrate a positive correlation between the system’s technical features and measurably improved patient outcomes.
Method
Schematic representation of the method; not approved for use in patients.
The product is not approved for commercial distribution.
Extensive Intellectual Property Portfolio

US 10,806,902 B2 (US, granted)
US 11,744,611 B2 (US, granted)
US 11,957,849 B2 (US, granted)
EP 3 328 478 B1 (EU, granted)
EP 3 746 167 B1 (EU, granted)
CN 111655321 B (China, granted)
AU 2016299343 B2 (Australia, granted)
AU 2019215680 B2 (Australia, granted)
BR 112018001895 B8 (Brazil, granted)
BR 112020015371 A8 (Brazil, granted)
422876 (India, granted)
547215 (India, granted)
JP 7315915 B2 (Japan, granted)
JP 7313698 B2 (Japan, granted)
PCT/EP2016/067785 (international patent application)
PCT/EP2019/051751 (international patent application)
201680044512.5 (China, pending)
References
- Barsky D, Radomislensky I, Talmy T, Gendler S, Almog O, Avital G. Association Between Profound Shock Signs and Peripheral Intravenous Access Success Rates in Trauma Patients in the Prehospital Scenario: A Retrospective Study. Anesth Analg. 2023 May 1;136(5):934-940. doi: 10.1213/ANE.0000000000006342. Epub 2023 Apr 14. PMID: 37058730. (18 Jan 2026; 14:45)
- Aldujeli A, Haq A, Tecson KM, Kurnickaite Z, Lickunas K, Bailey S, Tatarunas V, Braukyliene R, Baksyte G, Aldujeili M, Khalifeh H, Briedis K, Ordiene R, Unikas R, Hamadeh A, Brilakis ES. A prospective observational study on impact of epinephrine administration route on acute myocardial infarction patients with cardiac arrest in the catheterization laboratory (iCPR study). Crit Care. 2022 Dec 20;26(1):393. doi: 10.1186/s13054-022-04275-8. PMID: 36539907; PMCID: PMC9764590. (18. Jan. 2026, 15:00)
- https://www.cdc.gov/infection-control/hcp/intravascular-catheter-related-infections/summary-recommendations.html (18 Jan 2026, 18:19)Summary of Recommendations | Infection Control | CDC