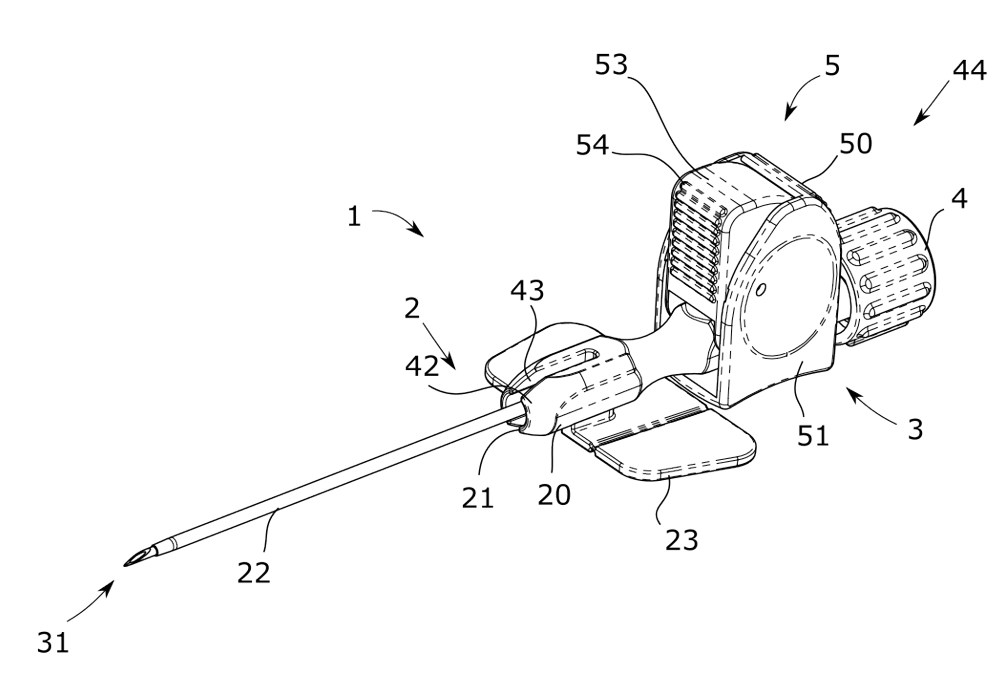
EbnetPIVC for First Puncture Success
Meaningful Improvements
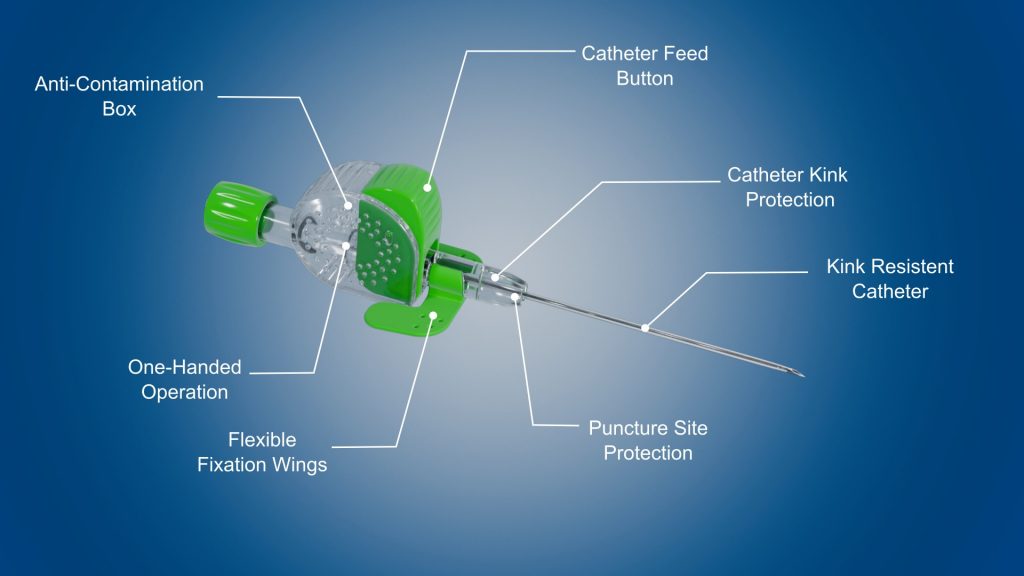
- Clear design for competitive manufacturability
- Intuitive handling for minimal training
- Stable handling for minimal risk of infection
- Easy one-handed operation for optimal use with ultrasound
- Catheter kink protection for secured infusion flow
- Optimization of usual workflows for rapid adoption
Currently under development, not yet cleared for commercial distribution.
For multiple future benefits and highly relevant study endpoints. A positive correlation between technical features and beneficial outcomes has to be shown.
Standard procedure versus EbnetPIVC (currently under development) for high-precision venous access
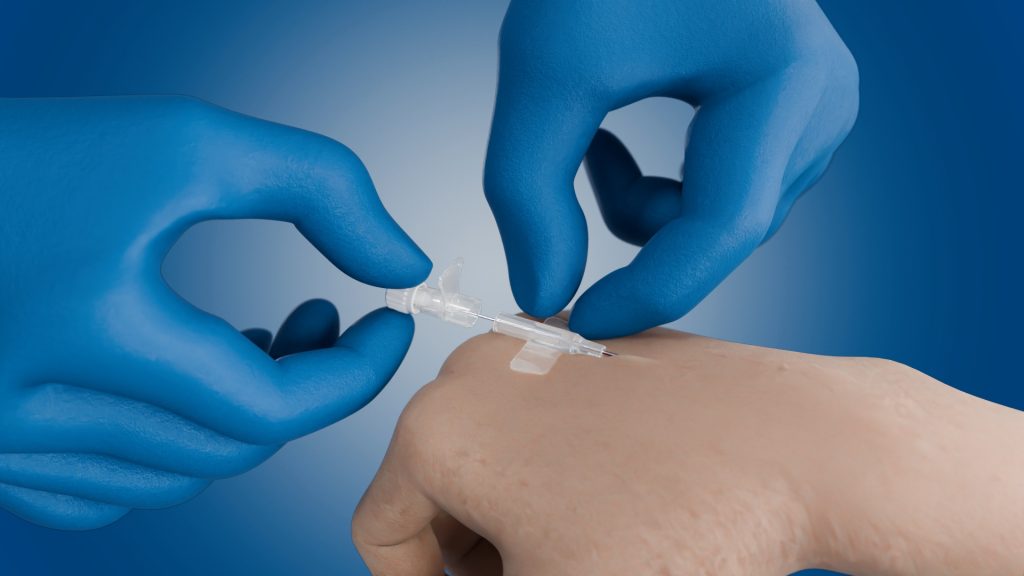
The standard procedure for venous access is imprecise, and the vein is often missed.
The catheter slides over the puncture needle in an uncontrolled manner leading to instability of the catheter system.
Veins often shift in the connective tissue when being punctured: “rolling veins”. Patient movements and environmental conditions further complicate the procedure.
The second hand is often required to stabilize the catheter system and can then no longer be used to fix “rolling veins”. It is also no longer possible to find veins with ultrasound since no hand is free.
A very shaky overall situation!
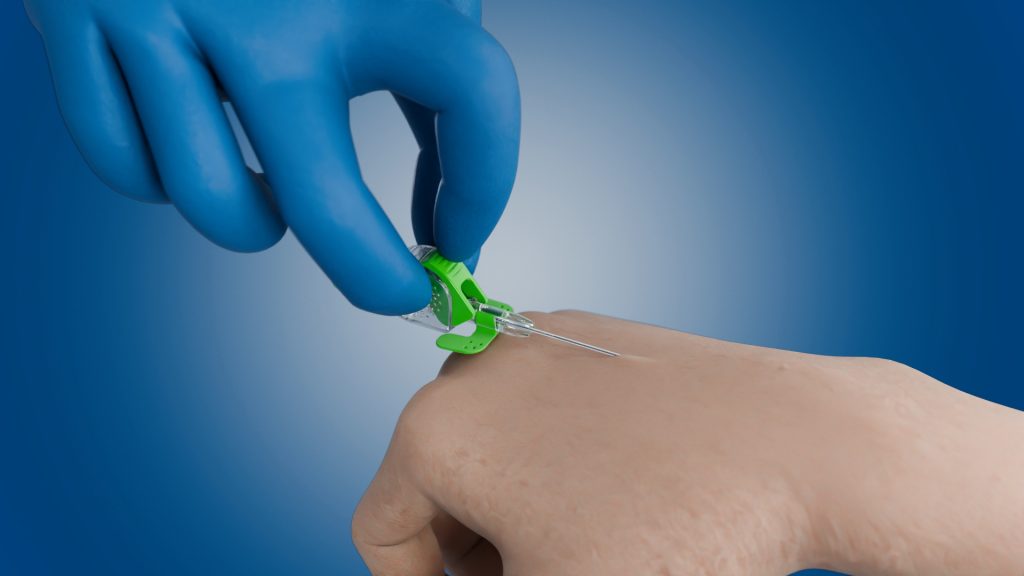
The EbnetPIVC (currently under development) is a stable catheter system and will enable simple and highly precise venous access with just one hand.
The easy-to-use feed mechanism will allow the catheter to be advanced very precisely into the vein.
The second hand is free to stabilize the anatomic structures or operate an ultrasound probe. The user can concentrate on the ultrasound monitor.
The risk of operating errors is minimized.
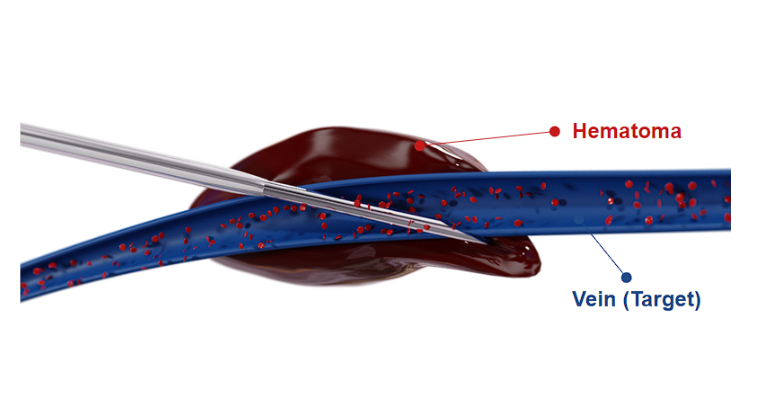
With the standard procedure, the vein is often missed. An unstable catheter system meets unstable anatomic structures.
Repeated painful punctures are required. Bruising, infection, and other serious complications are common.
Studies describe that medication often leaks from veins, rendering the medication ineffective and damaging tissue.

With the very stable EbnetPIVC (currently under development), the catheter can be placed precisely and gently.
In addition, the risk of puncturing the vein twice or slipping out of the vein with the catheter is minimized.
The EbnetPIVC (currently under development) is based on a proprietary platform technology for safe and hygienic catheter insertions for a variety of applications.
Extensive Intellectual Property Portfolio
20 2018 101 646.6 (Germany, utility protection model)
DE 10 2021 115 847 A1 (Germany, pending)
PCT/EP2019/057097 (international patent application)
PCT/EP2020/087404 (international patent application)
WO 2022/263550 A2 (international patent application)
19713743.3 (EU, pending)
20842219.6 (EU, pending)
17/040,342 (US, pending)
17/786,835 (US, pending)
201980028955.9 (China, pending)
202080095919.7 (China, pending)
202037041537 (India, pending)
2020-550830 (Japan, pending)
10-2020-7028923 (South Korea, pending)
Additional intellectual property filed (not yet published, options for numerous national/regional applications)
EbnetPIVC for First Puncture Success
Meaningful Improvements
- Clear design for competitive manufacturability
- Intuitive handling for minimal training
- Stable handling for minimal risk of infection
- Easy one-handed operation for optimal use with ultrasound
- Catheter kink protection for secured infusion flow
- Optimization of usual workflows for rapid adoption

Currently under development, not yet cleared for commercial distribution.
For multiple future benefits and highly relevant study endpoints. A positive correlation between technical features and beneficial outcomes has to be shown.
Standard procedure for venous access

The standard procedure for venous access is imprecise, and the vein is often missed.
The catheter slides over the puncture needle in an uncontrolled manner leading to instability of the catheter system.
Veins often shift in the connective tissue when being punctured: “rolling veins”. Patient movements and environmental conditions further complicate the procedure.
The second hand is often required to stabilize the catheter system and can then no longer be used to fix “rolling veins”. It is also no longer possible to find veins with ultrasound since no hand is free.
A very shaky overall situation!

With the standard procedure, the vein is often missed. An unstable catheter system meets unstable anatomic structures.
Repeated painful punctures are required. Bruising, infection, and other serious complications are common.
Studies describe that medication often leaks from veins, rendering the medication ineffective and damaging tissue.
EbnetPIVC (currently under development)
for high-precision venous access

The EbnetPIVC (currently under development) is a stable catheter system and will enable simple and highly precise venous access with just one hand.
The easy-to-use feed mechanism will allow the catheter to be advanced very precisely into the vein.
The second hand is free to stabilize the anatomic structures or operate an ultrasound probe. The user can concentrate on the ultrasound monitor.
The risk of operating errors is minimized.

With the very stable EbnetPIVC (currently under development), the catheter can be placed precisely and gently.
In addition, the risk of puncturing the vein twice or slipping out of the vein with the catheter is minimized.
The EbnetPIVC (currently under development) is based on a proprietary platform technology for safe and hygienic catheter insertions for a variety of applications.
Extensive IP Portfolio



20 2018 101 646.6 (Germany, utility protection model)
DE 10 2021 115 847 A1 (Germany, pending)
PCT/EP2019/057097 (international patent application)
PCT/EP2020/087404 (international patent application)
WO 2022/263550 A2 (international patent application)
19713743.3 (EU, pending)
20842219.6 (EU, pending)
17/040,342 (US, pending)
17/786,835 (US, pending)
201980028955.9 (China, pending)
202080095919.7 (China, pending)
202037041537 (India, pending)
2020-550830 (Japan, pending)
10-2020-7028923 (South Korea, pending)
Additional intellectual property filed (not yet published, options for numerous national/regional applications)
